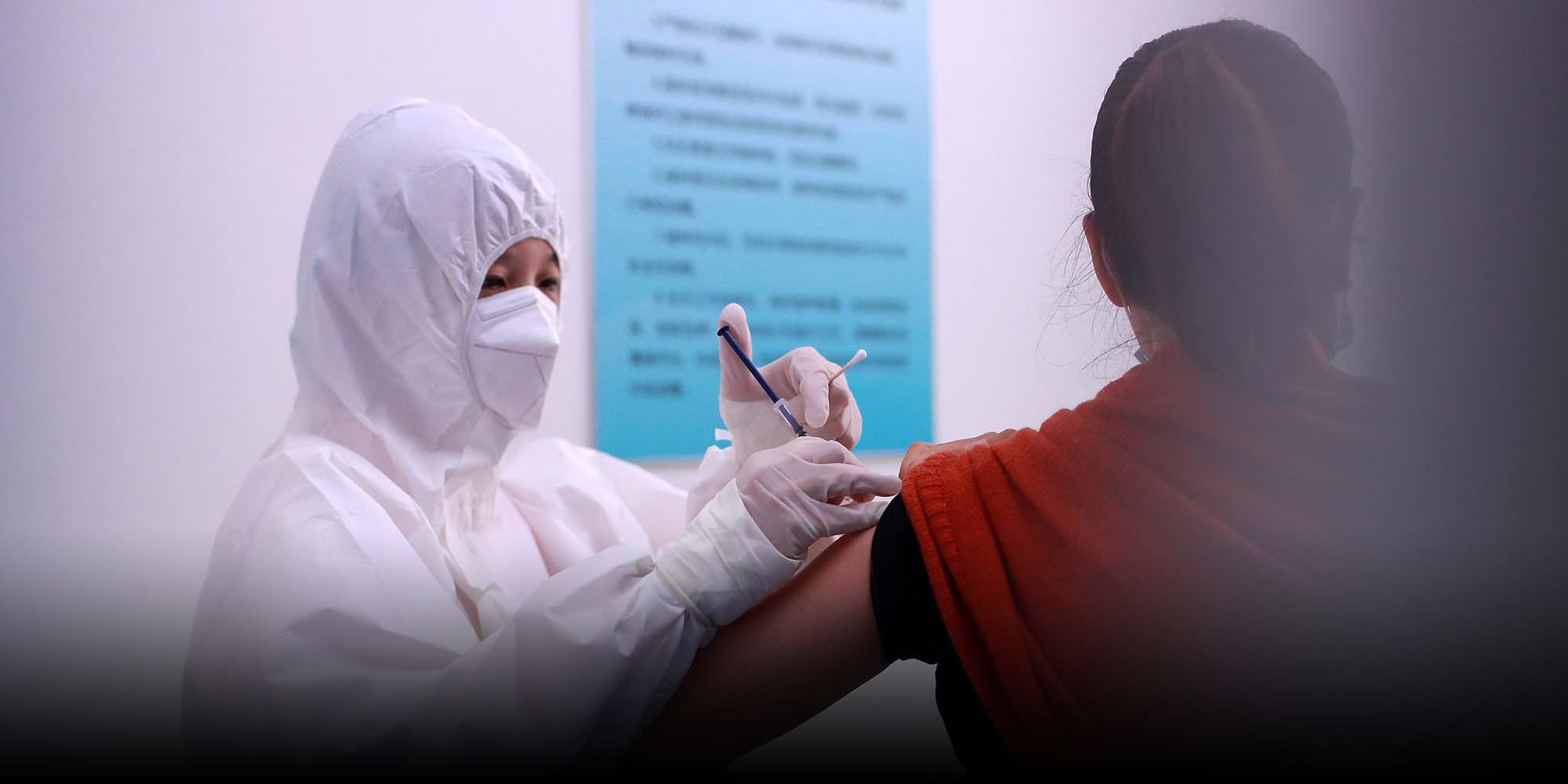
Despite Expected Supply Crunch, China Joins COVID-19 Vaccine Sharing Plan
China has joined an international initiative supporting fair distribution of COVID-19 vaccines across the globe, the country’s foreign ministry said Friday.
Aiming to distribute vaccines equally among participating countries according to their size, the so-called COVAX scheme is an attempt to avoid vaccine-producing countries prioritizing their own populations and allies over countries more in need.
The move came after the country’s leaders had vowed repeatedly to provide vaccine support to allied developing countries such as Cambodia and Laos. President Xi Jinping had also said during a health conference in May that once a COVID-19 vaccine becomes available in China, it “will be made a global public good.”
China is one of the front-runners in COVID-19 vaccine development. Chinese institutions are behind five of the 11 candidates that have so far been approved for large-scale human trials across the globe.
Since July, Beijing has approved at least three experimental vaccines for “emergency use,” which have been administered mainly to high-risk people such as frontline medical workers. Wu Guizhen, a senior official at China’s Center for Disease Control and Prevention, said last month that the country would have a COVID-19 vaccine ready by November or December this year, without referring to any specific candidate.
China joins at least 168 countries — potential vaccine producers Russia and the U.S. notably not among them — in signing up to the COVAX Facility initiative, which is led by the World Health Organization; Gavi, the Vaccine Alliance; and the Coalition for Epidemic Preparedness Innovations. It aims to make shots available first to “prioritized target groups” like frontline medical workers or the elderly, whom the WHO estimates to make up around one-fifth of a given country’s population.
Any vaccines approved for use will likely be in short supply initially. For example, Sinopharm, a state-owned pharmaceutical company that has two vaccines in the last stage of human trials, can make around 300 million doses per year, its CEO Liu Jingzhen said last month. If the company’s vaccines follow a two-dose schedule, as has been the case in clinical trials, yearly production would only be enough for about 10% of China’s 1.4 billion people.
Though the global supply of vaccines will likely rely on a variety of companies and countries, China deciding to share might present difficult decisions, Klaus Stöhr, an epidemic expert who previously worked at the WHO, told Nature on Thursday.
“The number of doses available in China will by far be too little to permit export unless a political decision is taken to ship vaccines overseas despite still-existing vaccine needs in China,” he said.
Editor: Kevin Schoenmakers.
(Header image: A volunteer is injected with an experimental coronavirus vaccine in Wuhan, Hubei province, April 15, 2020. People Visual)










